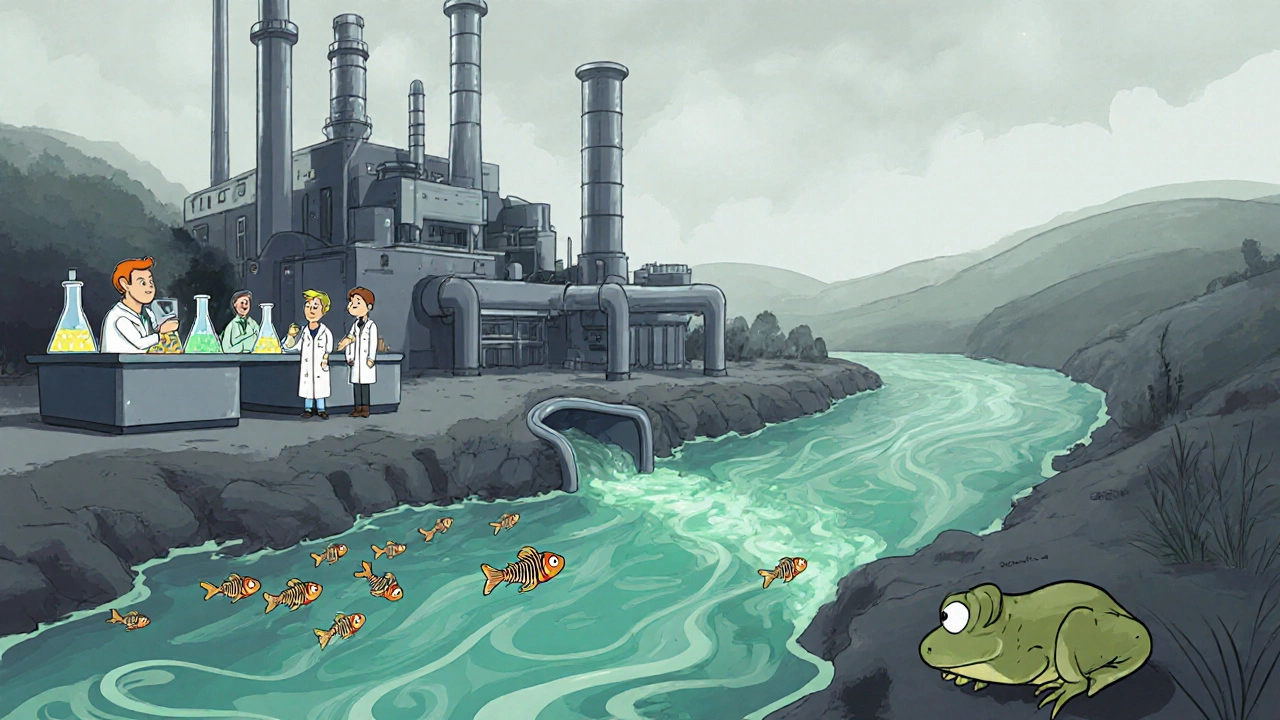
Enclomiphene Environmental Impact Calculator
How much enclomiphene is in your water source?
Enter the concentration of enclomiphene detected in your water supply to see the ecological risk level and potential impact on aquatic life.
Risk Assessment Results
Impact Reduction with Treatment
Advanced Oxidation: Reduces concentration by 50-80%
Granular Activated Carbon: Reduces concentration by 60-90%
Membrane Bioreactors: Reduces concentration by 70-95%
When you hear about Enclomiphene is a selective estrogen receptor modulator (SERM) used for male hypogonadism and fertility treatment, the focus is usually on its clinical benefits. Yet the chemicals that flow from factories into rivers and soils tell a different story. This article unpacks how the drug’s manufacturing chain leaves a footprint, what that means for ecosystems, and where the industry can turn to greener practices.
Key Takeaways
- Enclomiphene manufacturing releases hormone‑active residues that persist in wastewater.
- Even low‑level exposure can disrupt fish reproduction and affect downstream wildlife.
- Regulators such as the EPA and ECHA are beginning to set limits, but enforcement varies worldwide.
- Adopting green chemistry pathways and advanced wastewater treatment can cut emissions by up to 70%.
- Pharmaceutical companies that publish full life‑cycle assessments gain a competitive edge and reduce community backlash.
What Is Enclomiphene?
Enclomiphene is the trans‑isomer of the older fertility drug clomiphene. It works by blocking estrogen receptors in the brain, prompting a surge in luteinizing hormone (LH) and testosterone. The molecule’s chemical formula is C26H28ClNO, and its synthesis typically involves a multi‑step palladium‑catalyzed coupling followed by a stereoselective hydrogenation.
Because it’s a potent endocrine modulator, even trace amounts can influence hormone pathways in non‑target species. That’s why scientists monitor its fate once it leaves the production floor.
How Enclomiphene Is Made
The standard industrial route starts with 4‑chlorobenzophenone, which undergoes a Suzuki‑Miyaura cross‑coupling with a phenylboronic acid. The resulting biphenyl intermediate is then hydrogenated over a Raney nickel catalyst to give the desired trans‑isomer. Waste streams from each step contain solvents (toluene, ethanol), metal residues (palladium, nickel), and unreacted starting material.
Typical effluent concentrations-measured before any treatment-range from 5 to 30 µg L⁻¹ for enclomiphene and 10‑50 µg L⁻¹ for its precursor clomiphene. While these numbers sound tiny, they sit above the predicted no‑effect concentrations (PNECs) for many fish species.
Pathways Into the Environment
After leaving the plant, chemicals travel through three main routes:
- Wastewater discharge: Most facilities send effluent to municipal treatment plants, which are not fully equipped to strip out SERMs.
- Solid waste: Spent catalyst and filter media often end up in landfills, where leaching can introduce metals and organic residues into groundwater.
- Air emissions: Solvent vapors evaporate during distillation and may settle onto nearby soil.
Studies from the Netherlands and Sweden have detected enclomiphene in river water downstream of pharmaceutical clusters at concentrations up to 120 ng L⁻¹, well above the fish PNEC of 0.5 ng L⁻¹.

Ecological Impacts
Enclomiphene belongs to a broader group of endocrine disruptors that mimic natural hormones. In aquatic organisms, low‑dose exposure can cause:
- Reduced egg production in zebrafish.
- Altered sex ratios in amphibians.
- Decreased vitellogenin levels in male fish-an indicator of estrogenic activity.
These sub‑lethal effects cascade up the food chain, potentially influencing predator survival and ecosystem stability.
Regulatory Landscape
Both the US EPA and the European ECHA have begun to map pharmaceuticals in the environment. In 2023, the EPA released a draft “Pharmaceutical Manufacturing National Emission Standard” that proposes a limit of 0.1 µg L⁻¹ for selective estrogen receptor modulators, including enclomiphene. Europe’s REACH regulation already classifies SERMs as “substances of very high concern” when they display endocrine activity.
Compliance, however, varies. Some factories invest in advanced oxidation processes (AOPs) while others rely on conventional secondary treatment, leading to a patchwork of water‑quality outcomes.
Greener Alternatives & Mitigation Strategies
Switching to green chemistry routes can dramatically lower the environmental burden. Two promising avenues are:
- Biocatalytic hydrogenation: Enzyme‑based catalysts operate at ambient temperature, cutting energy use and eliminating precious metals.
- Continuous flow synthesis: Smaller reaction volumes reduce waste and improve product purity, meaning fewer by‑products need treatment.
On the waste‑management side, the most effective technologies are:
- Advanced oxidation (ozone/H₂O₂) that degrades SERMs into harmless fragments.
- Granular activated carbon (GAC) filters tuned for hormone removal.
- Membrane bioreactors (MBR) that combine biological degradation with high‑grade filtration.
When these methods are stacked, pilot studies show up to a 70 % drop in measurable enclomiphene concentrations before discharge.

Side‑by‑Side Comparison: Enclomiphene vs. Clomiphene
| Attribute | Enclomiphene | Clomiphene (racemic mix) |
|---|---|---|
| Water solubility (mg/L) | 0.03 | 0.07 |
| Half‑life in river water (days) | 12‑18 | 8‑12 |
| PNEC for fish (ng/L) | 0.5 | 1.2 |
| Observed downstream concentration (ng/L) | Up to 120 | Up to 250 |
| Endocrine activity (estrogenic) | High (ERα binding IC₅₀ = 45 nM) | Moderate (IC₅₀ = 120 nM) |
Checklist for Manufacturers
- Map every step of the synthesis and identify solvent‑heavy stages.
- Implement real‑time monitoring of effluent for enclomiphene using LC‑MS/MS.
- Upgrade to AOP or GAC treatment if concentrations exceed 0.1 µg L⁻¹.
- Conduct a life‑cycle assessment (LCA) and publish the results.
- Explore biocatalytic routes for hydrogenation to cut metal waste.
Future Outlook
The push for sustainable pharma is gaining momentum. By 2026, the International Council for the Harmonisation predicts that over 40 % of new drug‑manufacturing sites will embed circular‑economy principles. For enclomiphene, that means tighter bounds on emissions, more transparent reporting, and a shift toward greener synthesis.
Until those standards become universal, clinicians, patients, and advocacy groups must stay informed about the hidden costs of their medications. Knowing that a drug can affect fish in distant rivers adds a new dimension to the risk‑benefit conversation.
Frequently Asked Questions
Can standard municipal wastewater plants remove enclomiphene?
Most conventional plants only achieve 30‑50 % removal. Advanced oxidation or activated carbon is needed to consistently meet regulatory limits.
Is enclomiphene more harmful to the environment than clomiphene?
Both are persistent, but enclomiphene shows stronger estrogenic activity at lower concentrations, making its ecological risk profile slightly worse.
What green chemistry methods are viable for enclomiphene?
Biocatalytic hydrogenation and continuous‑flow synthesis are the two most promising, reducing solvent use by up to 60 % and eliminating heavy‑metal catalysts.
Do regulatory agencies require monitoring of enclomiphene in effluent?
The EPA’s draft standards would make it mandatory by 2027; Europe’s REACH already expects companies to report SERMs above 0.1 µg L⁻¹.
How can consumers influence better manufacturing practices?
Choosing pharmacies that source from manufacturers with published LCAs, supporting NGOs that push for stricter pharma waste policies, and asking doctors about drug‑sourcing can create market pressure for greener production.





In contemplating the ontological dimensions of pharmaceutical manufacturing, one must acknowledge that the very act of synthesizing enclomiphene transcends mere chemical transformation; it is an ethical negotiation between human health imperatives and ecological stewardship. The palladium‑catalyzed Suzuki‑Miyaura coupling, while elegant in its mechanistic choreography, begets palladium residues that persist in effluents unless rigorously reclaimed. Moreover, the subsequent Raney nickel hydrogenation introduces heavy‑metal contaminants that, when discharged, engender bioaccumulative pathways within aquatic trophic networks. From a systems‑thinking perspective, the emission of hormone‑active residues represents a perturbation of endocrine equilibria in downstream biota, manifesting as altered reproductive phenotypes. The documented concentrations of 5–30 µg L⁻¹, albeit ostensibly minute, eclipse predicted no‑effect concentrations for sensitive fish species, thereby constituting a non‑trivial ecological risk. Regulatory frameworks such as the EPA draft NMES and the European REACH directives acknowledge this risk yet suffer from heterogeneous enforcement, creating a patchwork of compliance whose efficacy remains empirically ambiguous. Green chemistry alternatives, notably biocatalytic hydrogenation, proffer a diminution of both energy input and metal usage, aligning with the principle of atom economy. Continuous‑flow synthesis further mitigates waste generation by curtailing reaction volumes and enhancing selectivity, thereby reducing the burden on downstream wastewater treatment. Advanced oxidation processes and granular activated carbon filtration, when deployed synergistically, have demonstrated up to a 70 % reduction in measurable enclomiphene concentrations, a quantitative testament to technological feasibility. Nonetheless, the philosophical impetus for such interventions resides not solely in regulatory compliance but in a broader moral calculus that weighs the benefits of male hypogonadism therapy against the costs borne by non‑human communities. Ultimately, the pursuit of sustainable pharmaceutics demands a convergence of rigorous life‑cycle assessment, transparent reporting, and an unwavering commitment to the principle that human health should not be attained at the expense of planetary health.
Wow, the chemistry behind enclomiphene is like a fireworks show in a lab-bright, intricate, and a bit messy when the sparks hit the environment. Those palladium and nickel residues are the party crashers nobody invited, but they keep showing up in the wastewater. Swapping in enzyme catalysts feels like replacing a gasoline car with a sleek electric ride-cleaner, quieter, and far less likely to pollute the river. And that continuous‑flow tech? It’s basically the assembly line of the future, slashing waste while cranking out pure product. Bottom line: the industry has a toolbox of greener tricks; it just needs to stop hiding the wrench.
Thank you for laying out the details so clearly. It’s reassuring to see specific mitigation strategies like AOPs and GAC filters mentioned, as they give us tangible steps to push for. I hope more manufacturers adopt these practices soon.
Sure, let’s all clap for the “green” pharma industry while they keep dumping hormones into our rivers. Nice try, regulators.
they r lyin, all that green talk is just a smokescreeen 4 big corp to keep profits up
Enclomiphene’s endocrine potency coupled with insufficient wastewater treatment yields a non‑trivial ecotoxicological load.
The data on altered sex ratios in amphibians underlines the necessity for upstream interventions. Implementing biocatalytic routes not only curtails metal waste but also aligns with a risk‑averse manufacturing ethos. Moreover, continuous‑flow systems can diminish by‑product formation, thereby reducing the load on downstream treatment. Stakeholders should prioritize these technologies as part of a comprehensive mitigation framework. The ecological stakes demand decisive, evidence‑based action.
Great insights! 🌿 It’s awesome to see science driving greener solutions. Keep it up! 👍
Really appreciate the deep dive into both the chemistry and the environmental angles. It’s clear that adopting advanced oxidation and GAC can make a huge difference. Let’s keep pushing for broader implementation across the industry.
Thanks for the sugestions! I think the key is to start small, like addng a GAC filter at one plant and see the results, then scale up. Typos be ware but the idea is solid.
The path forward is straightforward: use simple, proven methods like basic filtration and basic oxidation to cut down on hormone levels. If companies can’t afford fancy tech, they should at least implement the basics. This is not a luxury, it’s a necessity for protecting our water.