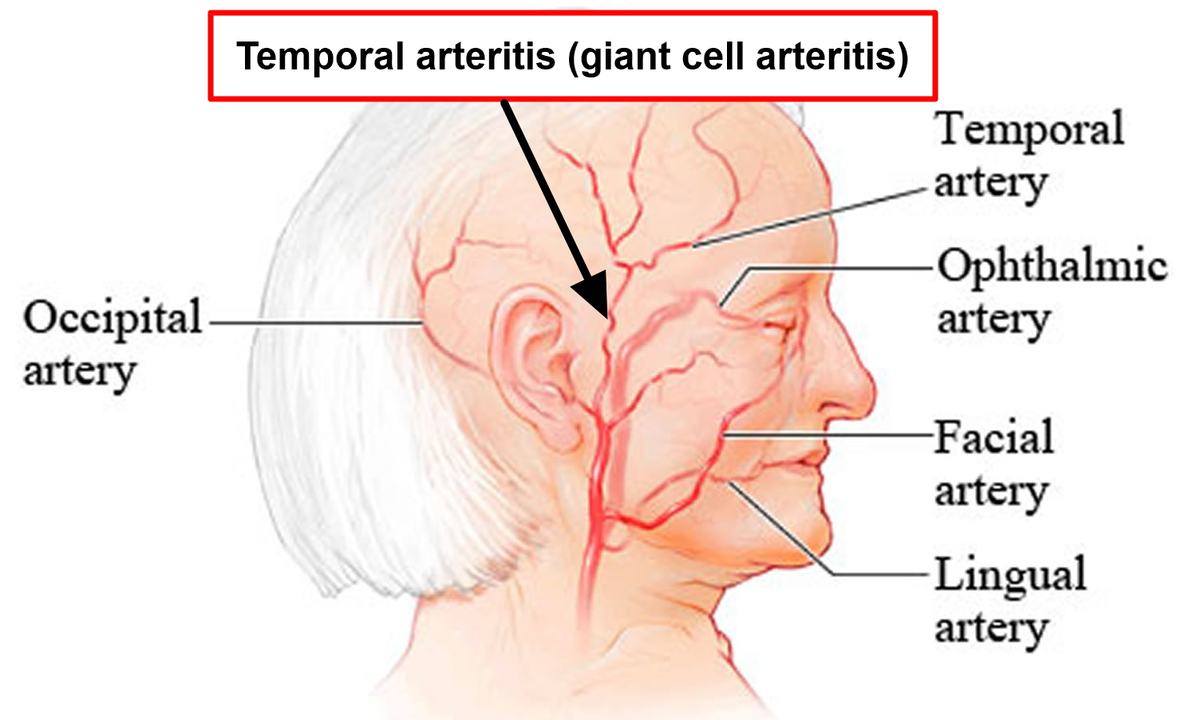
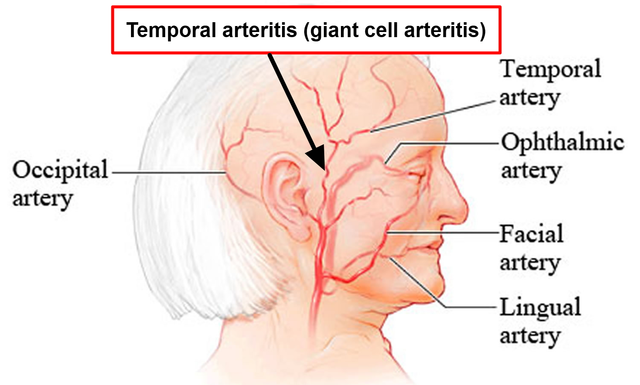
An Overview of Giant Cell Arteritis
Giant Cell Arteritis (GCA), also known as temporal arteritis, is an inflammatory disease that affects the large blood vessels, particularly those in the head, neck, and arms. It causes swelling and inflammation of the arteries, which can lead to severe headaches, vision loss, jaw pain, and even stroke. The exact cause of GCA is still unknown, but it is believed to be an autoimmune disorder in which the body's immune system mistakenly attacks its own arteries. In this article, we will explore Baricitinib, a promising new treatment option for GCA, and its potential role in managing this debilitating disease.
Understanding Baricitinib: What is it and How Does it Work?
Baricitinib is a selective Janus kinase (JAK) inhibitor, a class of drugs that work by blocking the activity of certain enzymes involved in the immune system's response to inflammation. By inhibiting these enzymes, Baricitinib can help reduce the inflammation associated with GCA, potentially alleviating symptoms and preventing further damage to the affected blood vessels. This medication has already been approved for the treatment of rheumatoid arthritis, another autoimmune disorder, in several countries, and researchers are now exploring its potential benefits for patients with GCA.
Baricitinib in Clinical Trials for Giant Cell Arteritis
So far, clinical trials for Baricitinib in the treatment of GCA have been promising. A recent Phase 2 study involving patients with newly diagnosed or relapsing GCA found that Baricitinib was effective in reducing inflammation and symptoms, such as headaches and jaw pain. The study also showed that Baricitinib allowed for a reduction in the use of corticosteroids, the current standard treatment for GCA, which can have significant side effects when used long-term. These results have led to the initiation of a Phase 3 trial, which is currently ongoing to further evaluate the safety and efficacy of Baricitinib in GCA patients.
Comparing Baricitinib to Current Treatment Options
As mentioned earlier, corticosteroids, such as prednisone, are currently the mainstay of treatment for GCA. While they are effective in reducing inflammation and relieving symptoms, their long-term use can lead to a range of side effects, including weight gain, osteoporosis, diabetes, and an increased risk of infections. Additionally, some patients may not respond well to corticosteroids, or may experience a relapse of their symptoms upon tapering the medication. In such cases, Baricitinib could potentially provide an alternative treatment option, with a different mechanism of action and a possibly more favorable side effect profile.
Potential Benefits of Baricitinib in Treating Giant Cell Arteritis
Given its mechanism of action and the results of clinical trials so far, Baricitinib has the potential to offer several benefits for GCA patients. Firstly, it may provide an effective treatment option for those who do not respond well to corticosteroids or experience relapses. Secondly, Baricitinib may help reduce the need for high-dose, long-term corticosteroid use, thus minimizing the risk of side effects associated with these medications. Lastly, Baricitinib may also offer a more targeted approach to treating GCA, as it specifically inhibits the enzymes involved in the inflammatory process, potentially leading to better outcomes and fewer side effects overall.
Conclusion: The Future of Baricitinib in Giant Cell Arteritis Treatment
As we continue to learn more about the potential role of Baricitinib in treating GCA, it is essential to keep in mind that this medication is still under investigation and not yet approved for this specific indication. However, the promising results from clinical trials thus far suggest that Baricitinib could become a valuable addition to the current treatment options for GCA. As research continues, we hope that Baricitinib will help improve the quality of life for those living with this challenging disease, offering a more targeted and potentially safer treatment option for patients in need.




Baricitinib’s JAK‑inhibiting action could indeed lessen the reliance on high‑dose steroids for GCA patients. By targeting specific inflammatory pathways, it offers a more focused approach, which may translate to fewer systemic side effects. It’s encouraging to see the phase‑2 data showing reduced headache frequency and jaw‑pain intensity. Continued monitoring for safety will be crucial as larger trials progress.
One might argue that the enthusiasm surrounding new JAK inhibitors sometimes eclipses the need for rigorous long‑term safety data. It’s a delicate balance, especially when patients have already endured the adverse effects of prolonged corticosteroid use. The current evidence, while promising, still leaves open questions about infection risk and cardiovascular implications. Nevertheless, the potential to taper steroids remains an attractive prospect.
Look, I’ve been following the updates on baricitinib for a while now and honestly it looks like a decent option for people who can’t handle the usual steroid regimen. The drug works by blocking those jak enzymes which are kinda like the messengers that tell your immune system to go haywire, so you end up with less inflammation in the arteries. The phase‑2 trial showed that patients reported fewer headaches and less jaw pain, which is a big deal because those symptoms can be pretty brutal. Plus, they were able to lower the steroid dosage, meaning fewer side effects like weight gain or bone loss. Of course, you still have to watch out for things like infections or blood clots, but that’s true for most immunosuppressants. Overall, it seems like a useful addition to the treatment toolbox, especially for those who relapse when steroids are tapered.
Baricitinib could be a game changer for GCA patients looking to cut down on steroids and feel better faster
Despite the hype, the pharmacokinetic profile of baricitinib raises concerns about off‑target JAK‑STAT modulation in vasculitic pathways.
While it is true that the kinetic parameters of baricitinib warrant close scrutiny, dismissing its utility outright overlooks the nuanced immunomodulatory benefits observed in recent phase‑2 outcomes. The drug’s selective inhibition of JAK1 and JAK2 pathways appears to attenuate the cytokine cascade that drives arterial wall inflammation, thereby offering a mechanistic rationale for its efficacy. Moreover, the reduction in corticosteroid exposure reported in the trial cohort suggests a tangible improvement in the risk‑benefit calculus for clinicians. Admittedly, the long‑term safety data remain incomplete, yet the early signals of decreased visual complications and fewer systemic adverse events are encouraging. In practice, a judicious, patient‑specific approach, possibly integrating baricitinib as a steroid‑sparing adjunct, could enhance therapeutic outcomes while mitigating the notorious side‑effects of chronic prednisone use.
Imagine a future where a GCA patient can walk out of the clinic with a smile, no longer shackled by the weight of daily steroid tablets-baricitinib may just paint that picture in vivid hues. Its targeted action whispers to the immune system, gently coaxing it back into harmony without the blunt force of high‑dose prednisone. The early trials sparkle with promise, showing not just symptom relief but a glimpse of restored quality of life. If subsequent studies confirm these findings, the therapeutic landscape could shift dramatically, offering clinicians a palette richer than ever before.
Baricitinib seems like a thoughtful addition to our GCA toolbox, especially for those who struggle with steroid side effects. The data so far suggest it can tame the inflammation while letting patients taper down prednisone more comfortably. It’s always reassuring when a new therapy aligns with what we already know about disease pathways, making the transition smoother for both doctors and patients.
Absolutely! 🎉 Adding a JAK inhibitor that dovetails with the underlying immunology feels like hitting the sweet spot. 🌟 It could also open doors for combination strategies, reducing the overall drug burden while still keeping disease activity in check.
The recent phase‑3 results will be pivotal in confirming whether baricitinib can reliably replace or reduce steroid therapy in GCA.
Indeed, the anticipation surrounding the upcoming data is palpable, and one must consider the multifaceted implications-efficacy, safety, cost‑effectiveness, and patient adherence-all of which intertwine to shape clinical decision‑making; moreover, the prospect of a steroid‑sparing agent could revolutionize standard care, potentially mitigating long‑term morbidities associated with glucocorticoids 🚀💊.
Baricitinib, as a selective JAK1/JAK2 inhibitor, enters the therapeutic arena with a promise to redefine the management of giant cell arteritis. Its mechanism, rooted in the attenuation of cytokine signaling, directly targets the inflammatory cascade that fuels arterial wall damage. The phase‑2 trial illuminated a reduction in hallmark symptoms such as throbbing headaches and debilitating jaw claudication, showcasing tangible patient benefit. More compelling, the study demonstrated a measurable decrease in cumulative prednisone dosage, hinting at a steroid‑sparing potential that could alleviate the burden of chronic glucocorticoid exposure. While the safety profile appeared acceptable in the short term, vigilant monitoring for opportunistic infections remains imperative, given the immunosuppressive nature of JAK inhibition. The upcoming phase‑3 data will undoubtedly clarify whether these early signals translate into sustained remission and improved visual outcomes. Clinicians are poised to weigh the nuanced trade‑offs between efficacy, safety, and cost, especially in the context of an aging patient population. Should the larger trial confirm durable disease control, baricitinib could become a cornerstone for patients who relapse or cannot tolerate high‑dose steroids. Moreover, the drug's oral administration offers a convenience advantage over injectable biologics, potentially enhancing adherence. Nonetheless, the need for long‑term data on cardiovascular risk, venous thromboembolism, and malignancy cannot be overstated. In the broader landscape, baricitinib exemplifies the shift toward targeted therapies that aim to modulate specific immune pathways rather than broadly suppressing immunity. This paradigm shift aligns with the evolving understanding of GCA as a heterogeneous disease with distinct molecular drivers. As we await more robust evidence, multidisciplinary collaboration will be essential to integrate baricitinib safely into treatment algorithms. Patients and physicians alike hope that this novel agent will usher in an era of reduced steroid dependence and better quality of life. In sum, baricitinib stands at the crossroads of promise and caution, demanding thoughtful appraisal as the scientific community moves forward.
The prospect of incorporating baricitinib into the therapeutic regimen for giant cell arteritis fills me with optimism, especially when considering the myriad challenges posed by long‑term steroid therapy. Patients often battle weight gain, osteoporosis, diabetes, and an increased susceptibility to infections, all of which can diminish their quality of life. Baricitinib’s targeted inhibition of the JAK‑STAT pathway offers a refined approach, potentially curbing inflammation while sparing patients from the harsh side‑effects of glucocorticoids. Early trial data have already hinted at improved symptom control, with notable reductions in headache severity and jaw pain, which are among the most debilitating manifestations of GCA. Moreover, the ability to taper steroids more rapidly could translate into faster functional recovery and less cumulative steroid exposure. From a clinician’s perspective, having an oral medication that can be administered conveniently enhances adherence, especially for older adults who may struggle with infusion schedules. The ongoing phase‑3 studies will be pivotal in confirming these promising signals, providing robust evidence on both efficacy and safety across diverse patient populations. If these results uphold the initial findings, baricitinb-sorry, baricitinib-might soon become a standard component of personalized treatment plans, empowering physicians to tailor therapy based on individual risk profiles and disease activity. In the meantime, continued vigilance, patient education, and shared decision‑making will be key to navigating this evolving therapeutic landscape.
While your enthusiasm is palpable, one must not overlook the latent shadows that accompany any novel immunomodulator. The JAK pathway, though alluring, intersects with a plethora of physiological processes, and its perturbation could usher unforeseen sequelae. History teaches us that today’s miracle can become tomorrow’s cautionary tale, especially when long‑term surveillance is lacking. Let us therefore temper our expectations with rigorous inquiry, ensuring that the promise of baricitinib does not eclipse the principle of patient safety.
Seems decent, but we’ll see 🤷♀️.
Baricitinib’s capacity to reduce corticosteroid load may be particularly advantageous for elderly GCA patients who are already at risk for bone demineralization. Careful patient selection and monitoring for infection remain essential components of any treatment plan incorporating a JAK inhibitor.
I agree, the risk‑benefit balance must be meticulously evaluated, especially given the immunosuppressive nature of JAK blockade.
Interesting how the field keeps evolving-new agents like baricitinib keep pushing the envelope, and it’ll be fascinating to watch the long‑term outcomes emerge 😊.
The mechanistic link between JAK inhibition and reduced arterial inflammation in GCA is a compelling area for further research.
It would be remiss to accept the burgeoning enthusiasm for baricitinib without a scrupulous examination of its pharmacovigilance data; indeed, the specter of off‑target effects looms large over any nascent therapeutic paradigm, and a cautious, evidence‑based stance remains the most prudent course.